The Philippines will conduct a clinical trial for Japan’s anti-flu drug Avigan on 100 COVID-19 patients, according to DOH
The Department of Health on Wednesday said that 100 COVID-19 patients in the Philippines will be given an Avigan, an anti-flu drug from Japan.
This, as DOH Undersecretary Maria Rosario Vergeire on Wednesday said that selected COVID-19 patients will be given the said drug as part of a clinical trial in treating the coronavirus disease 2019.
According to Usec. Maria Rosario Vergeire, the DOH will select hospitals that would join the clinical trial for the said drug, adding that the Department of Health will have a protocol on how to choose the patients. However, Vergeire underscored that the clinical trial for the said drug was “strictly voluntary” as the consent of the patients was required.
Usec. Maria Rosario Vergeire also said that the Philippines will begin conducting clinical trials “in the coming days” following the go-signal from Japan.
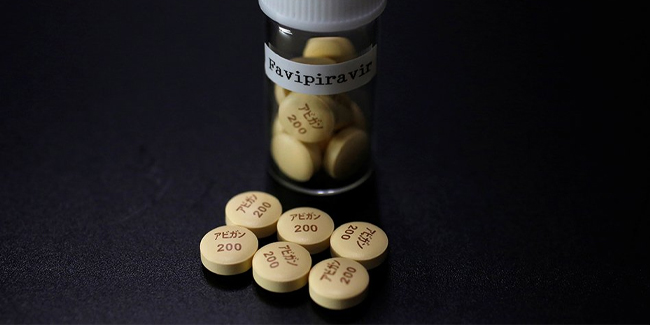
About Avigan
Avigan was also known for its generic name Favipiravir. It was an anti-flu drug that was developed by Toyama Chemical Co. in Japan.
Considering that this drug was an anti-flu drug, Japan already started conducting clinical studies after scientists suggested that Avigan had been effective in treating patients with coronavirus disease 2019 or COVID-19.
COVID-19 Tally in the Philippines
The Department of Health reported 199 new cases of COVID-19, with 93 new recoveries and 14 new coronavirus deaths as of 4:00 p.m. of May 5, 2020, bringing the total number of confirmed COVID-19 cases to 9,684, with 1,408 total recoveries and 637 total fatalities.
What can you say about this? Let us know!
For more news and updates, follow Philippine Newspaper on Facebook!
READ ALSO | Japan’s Avigan ‘Not Safe For Pregnant Women’, Says DOH
