The DOH warned the public that an anti-flu drug Avigan was “not safe for pregnant women”.
The Department of Health (DOH) warned the public about Avigan, an anti-flu drug from Japan, as this drug was “not safe for pregnant women”.
In a report from GMA Network’s news program 24 Oras, DOH Undersecretary Maria Rosario Vergeire on Wednesday said that the said drug will be tested if Avigan was effective in treating COVID-19 or coronavirus disease 2019, adding that there have been concerns about the drug’s effect on certain groups.
Citing information collected by the DOH, Usec. Maria Rosario Vergeire warned the public that Avigan was “not safe to use” for pregnant women.
Prior to this, DOH Undersecretary Maria Rosario Vergeire said that 100 COVID-19 patients will be given the said drug as part of a clinical trial in treating the coronavirus disease 2019.
Although the Philippines will start conducting clinical trials “in the coming days”, Usec. Vergeire underscored that the clinical trial for the said drug was “strictly voluntary” as the consent of the patients was required.
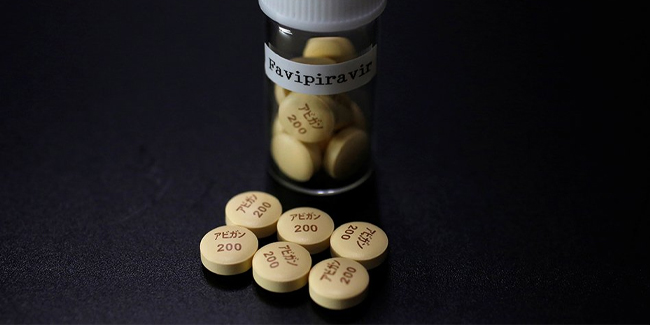
Avigan, also known for its generic name Favipiravir, was an anti-flu drug that was developed by Toyama Chemical Co. in Japan. Japan began conducting clinical studies following the suggestion of scientists that Avigan had been effective in treating COVID-19 cases.
The Department of Health reported 320 new cases of COVID-19, 98 new recoveries, and 21 new coronavirus deaths as of 4:00 p.m. of May 6, 2020, bringing the total number of confirmed COVID-19 cases to 10,004, with 1,506 total recoveries and 658 total fatalities.
More updates about this may be posted soon. Thank you for visiting Philippine Newspaper!
READ ALSO | PH To Run Clinical Trial For Avigan On 100 COVID-19 Patients, Says DOH
