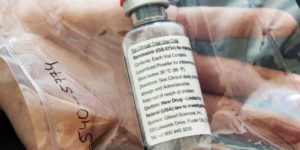
Remdesivir Has No Certificate of Product Registration — FDA
Remdesivir has no Certificate of Product Registration, according to FDA. The Food and Drug Administration (FDA) said that Remdesivir has no Certificate of Product Registration (CPR) in the Philippines. Remdesivir was an investigational drug that was currently being used on patients with coronavirus disease (COVID-19) in the Philippines. On Monday, the Food and Drug Administration … Read more