Japan planned to approve Remdesivir in treating patients with COVID-19, according to the Japanese government.
Japan on Thursday planned to approve the antiviral drug Remdesivir in treating patients with coronavirus disease 2019 or COVID-19, according to the Japanese government.
Aside from this, Japan was also targeting to approve the anti-flu drug Avigan this month. Japan will be the second country to approve the antiviral drug after the US Food and Drug Administration authorized Remdesivir for emergency use against severe cases of coronavirus disease 2019 or COVID-19.
In a statement from Yoshihide Suga, the top government spokesperson, the Japanese government will “swiftly approve” the antiviral drug if no problems were found.
Previously, Japanese Prime Minister Shinzo Abe said that the government was getting ready to have a go-signal to the antiviral drug.
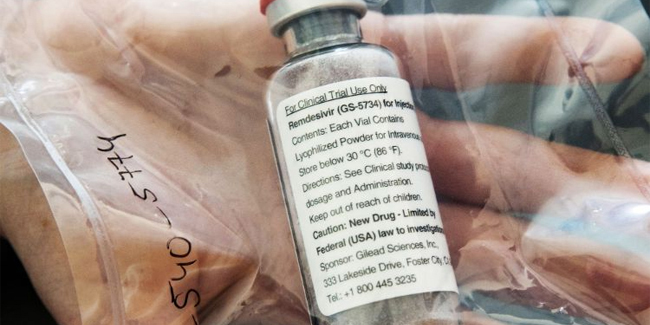
Gilead Sciences, the maker of the antiviral drug, donated 1.5 million vials of Remdesivir that can treat 100,000 – 200,000 COVID-19 patients in the United States through its government last Monday.
Daniel O’Day, the chief executive of Gilead Sciences, said that the company has been exporting Remdesivir for clinical trials and “for compassionate use” thousands of treatment courses, adding that there would be a significantly greater supply of the antiviral drug in the second half of 2020.
This antiviral drug was first authorized for emergency use by the US Food and Drug Administration.
What can you say about this? Let us know!
For more news and updates, follow Philippine Newspaper on Facebook!
READ ALSO | US FDA Approves Remdesivir, Issues Emergency-use Authorization
