Remdesivir has no Certificate of Product Registration, according to FDA.
The Food and Drug Administration (FDA) said that Remdesivir has no Certificate of Product Registration (CPR) in the Philippines.
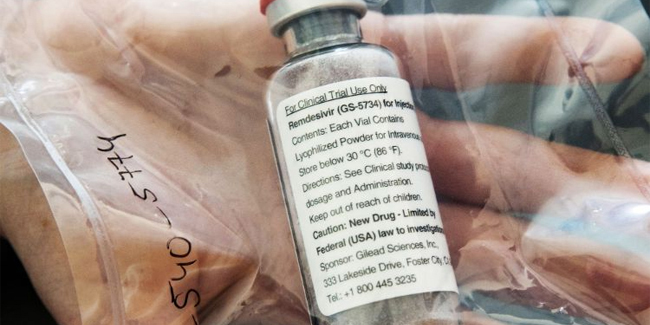
Remdesivir was an investigational drug that was currently being used on patients with coronavirus disease (COVID-19) in the Philippines.
On Monday, the Food and Drug Administration (FDA) clarified that the said drug has no Certificate of Product Registration (CPR) in the country.
According to the Food and Drug Administration, it granted Compassionate Special Permits (CSPs) for the said drug as requested by medical specialists for the treatment of COVID-19 patients.
The Food and Drug Administration also said that Remdesivir was used in adults and pediatric patients — particularly persons aged 12 years old and above — for the treatment of COVID-19 requiring hospitalization.
“It is clarified that a CSP is a special permit granted to physicians or hospitals to use investigational drugs or drugs which are not yet registered or in the process of registration here in the Philippines for the treatment of seriously ill patients,” the FDA said in an advisory.
READ ALSO: DOH Vows To Take Action vs Ivermectin Distribution In PH
The Food and Drug Administration explained that a Compassionate Special Permit can only be requested by doctors or hospitals, adding that the Compassionate Special Permit holder took full responsibility for the use and dispensing of the said drug.
Moreover, the Compassionate Special Permit holder must inform the patient of the investigational status of the said drug, and report to the Food and Drug Administration the results for every patient who received the said product.
The Food and Drug Administration also said that the permit was given only to qualified medical specialists or institutions who were authorized to use the said product to a specific number of patients, and was valid for one year.
For more news and updates, you may feel free to visit this site more often. You may also visit Newspapers.ph via our official Facebook page and YouTube channel.
