PSG Hospital will be responsible if President Duterte experienced adverse effects from Sinopharm vaccine, according to the FDA.
The Food and Drug Administration (FDA) said that the Presidential Security Group (PSG) Hospital will be responsible if President Rodrigo Duterte experienced adverse effects from Sinopharm COVID-19 vaccine.
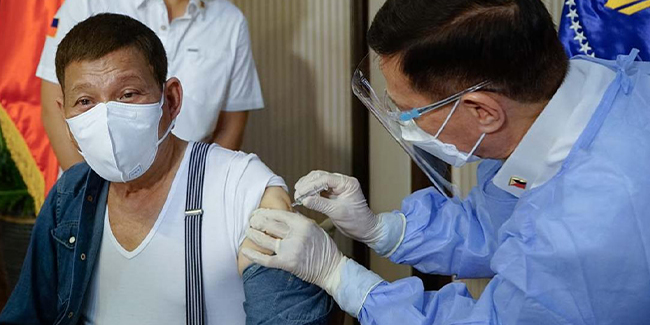
GMA News Online reported that the Presidential Security Group Hospital, which acquired the compassionate special permit (CSP) for China’s Sinopharm COVID-19 vaccine, will be responsible if the President experienced any adverse side effects.
In Lei Alviz’s report on GMA News’ “24 Oras”, FDA Director-General Eric Domingo said that the PSG Hospital director was the one who asked for the compassionate special permit from them.
“So ang nanghingi sa amin ng CSP is the director of the PSG Hospital. When we give a compassionate special permit, it is very clear that there is no assurance of safety, efficacy, and quality because it was not evaluated by the FDA. The PSG Hospital takes full responsibility for it,” Domingo said.
Domingo’s statement came after President Duterte received his first dose of China’s Sinopharm COVID-19 vaccine on Monday night. The said vaccine was administered by no less than Health Secretary Francisco Duque III.
READ ALSO: President Duterte Receives First Dose Of Sinopharm COVID-19 Vaccine
A compassionate use permit only allowed for legal administration of COVID-19 vaccine in the country and wasn’t tantamount to the Food and Drug Administration endorsing the product’s efficacy and safety.
So far, Domingo said that there were two to three companies that have expressed their intent to apply for the emergency use authorization (EUA) of the COVID-19 vaccine developed by China’s Sinopharm Group.
However, those companies have yet to submit requirements to the Food and Drug Administration — including proof that they’re the official supplier and distributor of the said vaccine in the Philippines.
For more news and updates, you may feel free to visit this site more often. You may also visit Newspapers.ph via our official Facebook page and YouTube channel.
