The generic version of Paxlovid will be distributed in government hospitals.
BEXOVID — The generic and more affordable version of Pfizer’s Paxlovid will be distributed in government hospitals in the Philippines.
This, after the Philippine Food and Drug Administration (FDA) has granted the Department of Health’s application for a compassionate special permit (CSP) for Bexovid.
FDA Director-General Dr. Oscar Gutierrez said that Bexovid would be distributed to government hospitals in the Philippines.
“Dahil ito po ay generic, ito po ay inaasahan po na mas mura ang cost of treatment compared to Paxlovid,” Gutierrez reported in President Rodrigo Duterte’s “Talk to the People” public briefing. “The CSP po is given to DOH and the DOH will distribute this to government hospitals once it is supplied by Biocare Lifescience.“
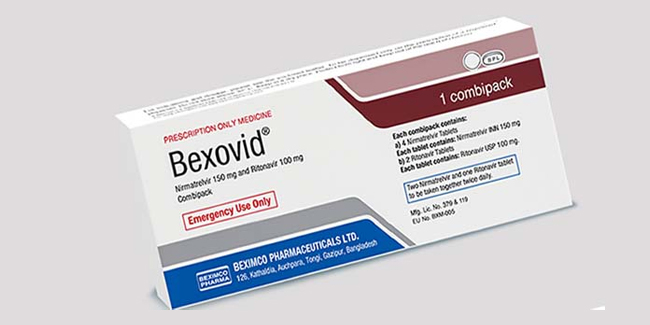
Bexovid became the world’s first generic version of Paxlovid made by Pfizer. Its generic name is Nirmatrelvir and Ritonavir.

Based on Gutierrez’s presentation, Bexovid was made by Beximco Pharmaceuticals and will be distributed in the country by Biocare Lifescience.
The treatment was said to reduce the risk of hospitalization or death by 89 percent if given within three days of the onset of symptoms and by 88 percent if given within five days.
According to Gutierrez, the Food and Drug Administration met with Pfizer representatives last January 7 and the company was likely to file an application for an emergency use authority (EUA) in the country in the last week of the month.
READ ALSO: Faberco Assures Access To Molnupiravir In PH
At present, the Food and Drug Administration had given EUAs for the COVID-19 anti-viral drugs Molnupiravir (Molnarz) and Casirivimab and Imdevimab (Ronapreve).
It has a CPR (certificate of product registration) for Remdesivir or Covifor. The registration allowed the marketing or free distribution of the said medicine.
Gutierrez also said that the CPR will eliminate the distribution of Remdesivir in the black market for steep prices.
In addition, Gutierrez said that the Food and Drug Administration had received two product applications for self-administered COVID-19 test kits in the country.
The government was looking at allowing self-test kits following the steep increase in coronavirus cases in the country over the past two weeks.
For more news and updates, you may feel free to visit this site more often. You may also visit Newspapers.ph via our official Facebook page and YouTube channel.
