Clinical trials of Avigan in the Philippines will start on August 10, according to DOH.
The clinical trials of Japan’s anti-viral drug Avigan in the Philippines would begin on August 10, according to Health Undersecretary Maria Rosario Vergeire.
This, as the quest for the possible treatment for coronavirus disease (COVID-19) continued worldwide.
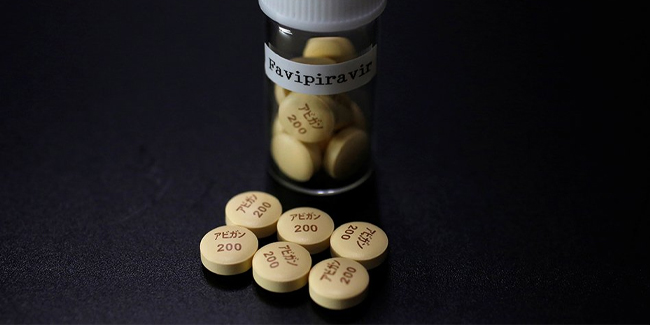
Vergeire on Friday said in an interview on GMA Network’s Unang Hirit that all was set for the clinical trial to start after the Philippines had received around 199,000 tablets of Avigan from Japan for the clinical trials.
She also said that they had already identified the hospital site where the said drug will be used.
Other Stories
- Ilagan City Mayor, Wife, Son Test Positive For Coronavirus Disease
- Here’s Why Rica Peralejo Loves And Hates Pandemic
- Only One Province In PH Remains COVID-19-Free
Avigan was known as the brand name of favipiravir, was developed by Fujifilm Toyama Chemical, and was approved for use last 2014 in Japan. However, the said drug was only approved for use in flu outbreaks in Japan and wasn’t being addressed by existing medications effectively.
Moreover, the said drug wasn’t available on the market and could only be manufactured and distributed at the request of the government of Japan.
The clinical trials of the said drug came amid the increasing number of COVID-19 infection in the country, which was already identified as a “virus hotspot” in Southeast Asia, overtaking Indonesia on Thursday with over 119,000 COVID-19 infections.
What can you say about this? Let us know in the comments below!
For more news and updates, you may follow Philippine Newspaper on Facebook!
