Remdesivir was still part of the ongoing clinical trial in the Philippines, according to the Department of Health.
The Department of Health on Thursday evening said that the antiviral drug Remdesivir was still part of the ongoing clinical trial in the Philippines.
This, as Undersecretary Maria Rosario Vergeire said in a statement that the antiviral drug was one of the investigational drugs that were included in the Solidarity Trial of the World Health Organization (WHO).
“Solidarity Trial”
Usec. Maria Rosario Vergeire that the solidarity trial was led by the World Health Organization and studied the effectiveness of four different drugs — Remdesivir, Hydroxychloroquine, Lopinavir with Ritonavir, and Lopinavir with Ritonavir plus Interferon beta-1a — for the treatment of COVID-19 or coronavirus disease.
As per the Department of Health, Five hundred (500) patients with coronavirus disease 2019 or COVID-19 in the Philippines will be part of WHO’s solidarity trial.
About Remdesivir
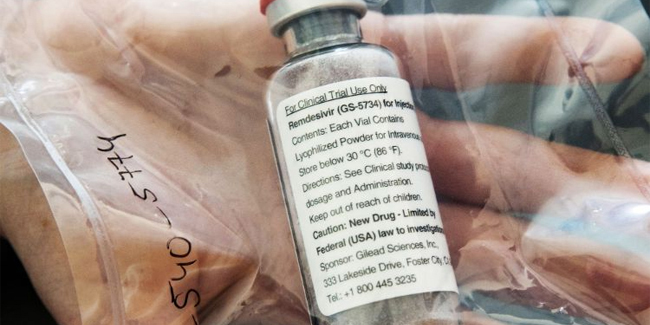
Usec. Vergeire mentioned that this antiviral drug had early evidence that it may reduce the duration of recovery for COVID-19 patients, adding that the shipment of the antiviral drug has already arrived in the Philippines and was being distributed to the country’s study sites.
Remdesivir was first authorized for medical use by the United Stated Food and Drug Administration and was recently approved in Japan. Both US and Japan will distribute this antiviral drug to patients with severe symptoms of coronavirus disease 2019 or COVID-19.
This antiviral drug was manufactured by Gilead Sciences Inc.
More updates about this may be posted soon. Thank you for visiting Philippine Newspaper!
