Canada approved Remdesivir for use against the coronavirus disease.
Canada on Tuesday had approved the antiviral drug Remdesivir in order to treat people with severe symptoms of coronavirus disease (COVID-19).
This, as the Canadian health ministry said that Remdesivir was the first drug that Health Canada had authorized for the treatment of coronavirus disease.
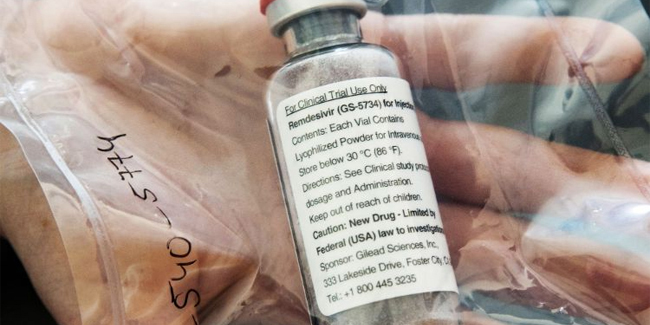
At least two (2) major studies in the United States had shown that the said drug could reduce the duration of hospital stays for patients with coronavirus disease.
Washington first authorized the said drug for “emergency use” – which was originally intended to treat patients with Ebola virus – last May 1, then followed by other countries including South Korea and Japan.
Other Stories
- BLACKPINK New Single: BLACKPINK Collabs With Selena Gomez?
- Celebrities Post Black and White Photos – “Challenge Accepted”
- BLACKPINK Lisa As Official Endorser Of New vivo Product? [PHOTO]
Canada said that Remdesivir could be used on patients with coronavirus disease who had pneumonia and need an extra oxygen in order to help them breathe. In addition, the doses of Remdesivir used in Canada would be made by a unit of Gilead Sciences.
Based on a report on AFP News, the European Commission authorized the use of the antiviral drug in early July in order to treat patients with coronavirus disease.
Gilead Sciences was a pharmaceutical company in the United States that developed the antiviral drug.
What can you say about this? Let us know in the comments below!
For more news and updates, you may follow Philippine Newspaper on Facebook!
